Modernizing a legacy LIMS (Laboratory Information Management System) is no longer optional for genomics and diagnostics organizations; it's a matter of operational survival.
A 2024 Deloitte survey found that 92% of genomics labs cite LIMS modernization as their top digital transformation priority, yet 63% report failed attempts or major disruptions during migration, primarily due to poor planning, compliance gaps, and lack of architectural foresight.
The reality is simple:
- Legacy LIMS systems were not designed for today’s volume of sequencing data, integration requirements, or compliance expectations.
- Modern genomics workflows require cloud-native infrastructure, robust auditability, and streamlined interoperability across lab, compute, and clinical systems.
Yet many labs approach modernization like a simple IT upgrade. They treat it as “moving data to a new platform,” rather than rebuilding the foundation for an evolving genomic enterprise.
This is where projects fail and costs explode.
In this guide, we’ll walk through:
- Why LIMS modernization is uniquely difficult in genomics
- The core architectural and compliance principles you must get right
- The core architectural and compliance principles you must get right
- A step-by-step modernization roadmap used by high-performing labs
- Common pitfalls and how to avoid them
- How to decide whether to build, buy, or adopt a hybrid model
Why Legacy LIMS Systems Break in Genomics
LIMS platforms originally emerged to manage sample tracking, lab inventory, and operational workflows. In traditional clinical labs, they handled predictable data types and linear processes.
Genomics changes everything:
- Massive file sizes: FASTQ, BAM/CRAM, VCF, QC reports
- Complex pipelines: multi-stage computational workflows, reproducibility requirements
- Interoperability demands: sequencers, bioinformatics tools, EHR systems, payer systems, reporting tools
- Compliance scope: HIPAA, CLIA, CAP, SOC 2, GDPR and genetic privacy regulations
- Scientific evolution: assays, pipelines, and interpretation logic change continuously
Most legacy LIMS systems cannot adapt to these dynamics. Their data models are rigid, integration layers are brittle, and auditability is often weak.
The Three Hidden Risks of LIMS Modernization
1. Operational disruption
A lab cannot “pause” operations. If your modernization effort disrupts sample tracking, instrument connectivity, or reporting, it impacts turnaround time, revenue, and patient outcomes.
2. Compliance failure
If your new architecture fails a HIPAA risk assessment, SOC 2 audit, or CLIA/CAP validation, the project is not just delayed, it may become unusable in clinical environments.
3. Vendor lock-in (or internal lock-in)
Many labs modernize by purchasing a COTS LIMS and customizing it. They later discover that:
- Customization costs balloon,
- Integration timelines stretch,
- And switching platforms becomes nearly impossible.
Alternatively, labs build custom systems and become dependent on a small internal engineering team. When key developers leave, the platform becomes unmaintainable.
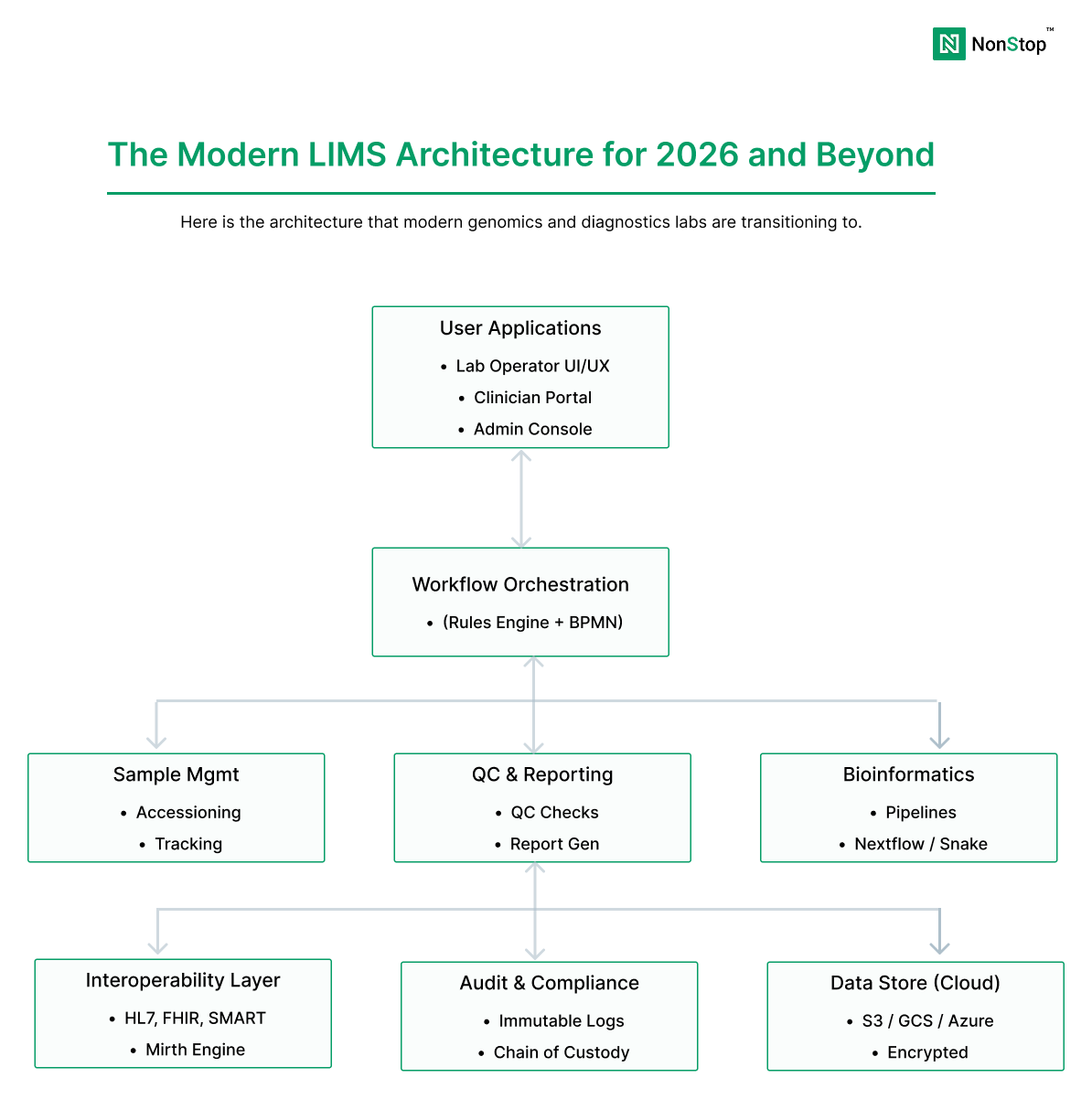
The Modern Genomics LIMS Architecture: What “Good” Looks Like
A modern LIMS platform for genomics typically separates concerns into distinct layers:
- Lab Operations Layer: sample accessioning, QC, chain of custody, batching, instrument integration
- Bioinformatics Layer: pipeline orchestration, compute, reproducibility, outputs
- Interpretation & Reporting Layer: annotation, scoring, clinical interpretation, report generation
- Integration Layer: EHR, billing, partner portals, CROs, data exchange standards (HL7/FHIR)
- Compliance & Observability Layer: audit logs, access controls, monitoring, incident response readiness
This modular architecture allows labs to modernize incrementally, replace components without re-validating the entire system, and scale compute and storage independently.
A Practical LIMS Modernization Roadmap (Step-by-Step)
Step 1: Define your scope and modernization goals
Start by clarifying what you are modernizing and why.

Step 2: Inventory workflows and data flows (before you touch code)
Most modernization failures occur because teams underestimate workflow complexity.
Document:
- Sample lifecycle workflows (accessioning → QC → batching → sequencing → analysis → reporting)
- Instrument and sequencer integrations
- Pipeline orchestration and compute dependencies
- EHR and partner integrations
- Manual workflows that exist today
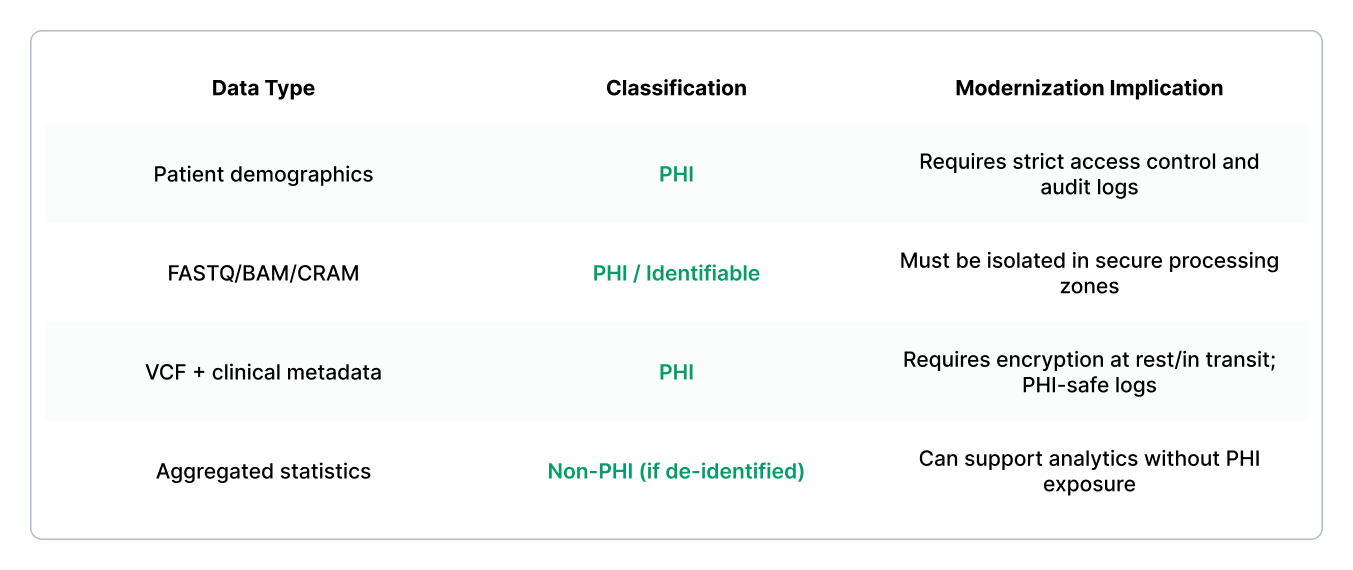
Step 3: Classify data and establish PHI boundaries
A genomics LIMS almost always contains PHI because sample identifiers, patient metadata, and genomic files can be re-identifiable.
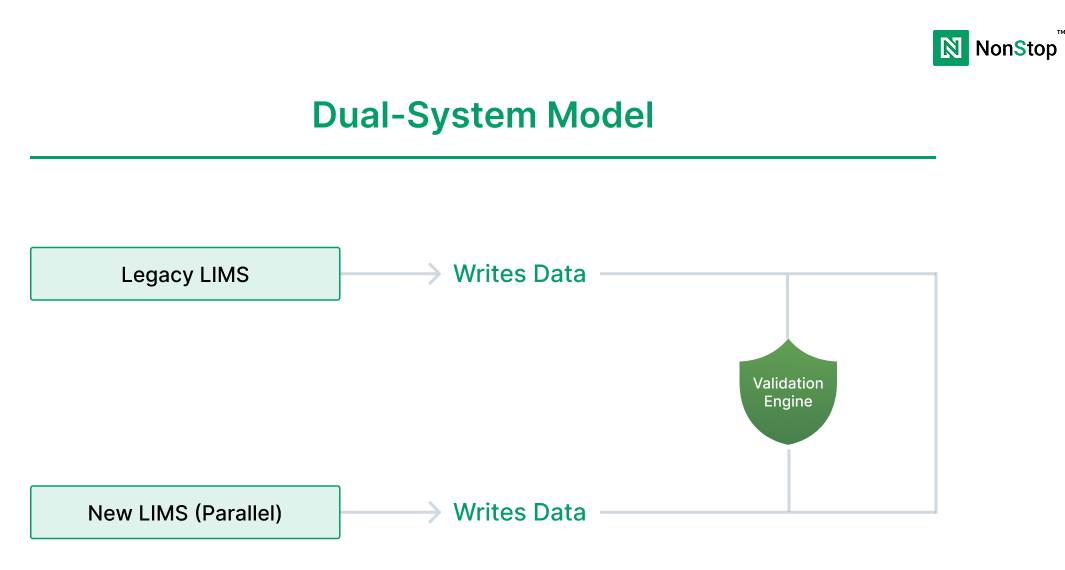
Step 4: Choose your modernization strategy (build, buy, or hybrid)
Most genomics organizations end up in a hybrid model: buy core utility functions, build differentiated workflows and integrations.
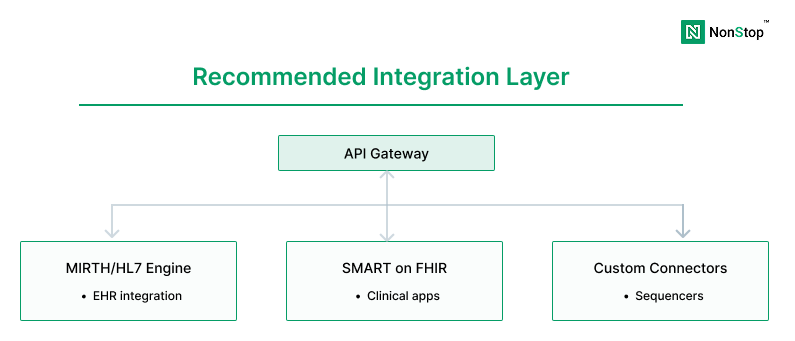
Step 5: Design for interoperability (EHR + instruments + partners)
Modern LIMS platforms must integrate seamlessly with:
- Sequencers and instruments (Illumina, Thermo, etc.)
- Pipeline orchestration systems (Nextflow, Cromwell, Airflow)
- Clinical EHR systems (Epic, Cerner) via HL7/FHIR
- Billing and payer systems
- Partner portals and CRO data exchange
The integration layer is often where modernization either succeeds or fails.
Step 6: Implement compliance-by-design (not compliance-after)
Compliance is not a checklist you add at the end. You must architect for:
- Role-based access control (RBAC) and least-privilege IAM
- Centralized, immutable audit logs
- Encryption at rest and in transit
- Secure pipeline execution zones
- PHI-safe observability (no PHI in logs)
- Validation protocols (CLIA/CAP where applicable)
Step 7: Execute migration in phases (avoid big-bang cutovers)
Big-bang cutovers are one of the biggest causes of lab disruption.
Preferred phased approach:
- Phase 1: Parallel run for sample tracking + basic workflow
- Phase 2: Add pipeline orchestration + compute integration
- Phase 3: Add reporting + EHR integration + advanced automation
- Phase 4: Decommission legacy systems only after validated stability
Common Mistakes That Derail LIMS Modernization
- Skipping workflow discovery: teams underestimate real lab behavior
- Ignoring PHI boundaries: leads to failed risk assessments and re-architecture
- Not planning data migration rigorously: data quality issues surface late
- Building monoliths: locks teams into inflexible systems
- Underestimating interoperability: integration delays dominate project timelines
- No structured validation plan: causes compliance failures and deployment delays
Conclusion
A successful genomics LIMS modernization initiative requires more than a new UI or a database migration. It demands a full rethinking of architecture, compliance, interoperability, and operational workflows.
The labs that modernize successfully do so by:
- treating modernization as a product transformation, not an IT upgrade,
- architecting compliance and auditability from the start,
- planning phased migration to avoid lab disruption,
- and building modular systems that can evolve as science advances.
If you’re considering LIMS modernization in genomics, it’s worth approaching it with the same rigor you apply to assay validation: clear requirements, structured execution, and an architecture built for scale, change, and compliance.



