The True Cost of Building vs. Buying Genomics Software: A Complete Decision Framework for Genomics Leaders in 2026
If you are a CTO, VP of Bioinformatics, or a Lab Director in the life sciences today, you are facing one of the most stressful capital allocation decisions of your career. The choice between building a custom software solution (a homegrown LIMS or CDS system) and licensing a commercial-off-the-shelf (COTS) solution feels like a binary, high-stakes gamble.
The real anxiety isn't the upfront cost. It's the unpredictable chaos that follows: the hidden maintenance bills, the crippling technical debt, the panicked scrambles before a HIPAA or GxP audit, and the certainty that whatever you choose today will be obsolete tomorrow.
The simple Build vs. Buy analysis is obsolete. The only question that matters is: How do we achieve the lowest, most predictable Total Cost of Ownership (TCO) over the next seven years while maintaining the flexibility to integrate the next scientific breakthrough?
This is not a marketing pitch; it's an open conversation from a team that has spent over a decade engineering and scaling platforms in this highly regulated space. We're going to walk through the predictable, catastrophic costs of both paths and outline the only viable strategy. This targeted, hybrid model guarantees compliance and keeps your capital focused on scientific innovation, not on firefighting IT debt.
I. The Core Problem: Unpredictable Chaos, Not Just Cost
Before we talk about budgets, let's talk about the friction you already feel. Genomic and precision medicine platforms are uniquely complex because they must bridge three fundamentally different worlds: the lab (LIMS), the computation (Bioinformatics), and the clinic (EHR).
Server costs don't drive the high TCO; the human and compliance costs of this constant disconnect drive it.
The Critical Path: From Sequencer to Point-of-Care
Think about a single patient sample. Its data must flow through the entire system without error, without manual intervention, and with an unbroken chain of custody:
- Secondary Analysis: Raw sequencer data is processed (aligned, variant called). This demands a robust, containerized environment to ensure results are reproducible and auditable, a non-negotiable requirement for GxP standards.
- Tertiary Analysis: The VCFs are annotated, interpreted, and passed through your proprietary scoring algorithms. This is your company's intellectual property (IP)
- Clinical Integration: The final interpretive report must be safely and seamlessly deposited into the patient's Electronic Health Record (EHR) and accessible by the physician. This is often the most expensive point of failure.
If your system relies on manual data transfer, or if your LIMS and EHR are fighting over communication standards (like HL7 vs. FHIR), you are not just inefficient; you are operating with systemic revenue and compliance risk.
II. Option 1: The Trap of Building It Yourself
The Build decision starts with the promise of perfect customization and low initial cost. It invariably ends in chaos. Why? Because you end up paying the full price for specialized talent and architectural debt, and you absorb 100% of the compliance risk.
The Talent Nightmare: Specialized Engineers and Knowledge Loss
You don't just need a software engineer; you need a Bioinformatics Software Engineer, a highly specialized, expensive hybrid role.
- The Annual Salary Trap:
- A single Bioinformatics Engineer or Developer in the U.S. can average between $89,000 and $131,053 annually.
- A Full-Stack Engineer with specific Healthcare startup experience averages around $153,000 per Year.
A minimal core team of three engineers costs you $400,000 to $600,000+ annually in salary and benefits alone. This figure is predictable.

- The Unpredictable Cost: Knowledge Retention
The real TCO spike occurs when your key developer quits. Homegrown LIMS systems become liabilities because they depend entirely on siloed development knowledge. When the creator of your custom analysis pipeline leaves, you are left with technical debt, zero documentation, and no support. Your lab's ability to adapt to new assays or fix a critical bug is suddenly hostage to a single person.
- The Architecture Tax: Maintenance, Microservices, and Unpredictable Bills
Custom code means you own the entire operational stack, leading to predictable cost inflation:- Infrastructure Sprawl: While microservices offer great agility, they are financially demanding. This architecture can increase your infrastructure costs by 50–100% compared to a consolidated system, due to increased debugging complexity and orchestration needs. For a high-compute genomics platform, this difference is substantial and persistent.
- Lack of Reliability: Homegrown LIMS often suffer from limited reliability and un-scalable design. Debugging falls onto internal staff who lack the time or deep expertise to fix persistent issues, leading to system downtime, reduced performance, and massive operational costs.
- The Compliance Burden: When GxP and HIPAA Audits Become Your Full-Time Job
In life sciences, compliance is not a feature; it's the foundation. When you build, you inherit 100% of this recurring, mandatory cost:- GxP Validation: The necessary process of validating a new analytical system to GxP standards should cost less than 5% of the total budget, but it frequently balloons to 25% or 30% without a proven, repeatable validation process. You are paying your highly paid scientists to become compliance auditors.
- SOC 2 Certification: Necessary for building trust with partners. Achieving and maintaining SOC 2 certification can average between $30,000 and $150,000 annually, including audit fees and over 100 hours of internal team effort.
- The Reality: Home-built systems often lack robust logging, standardized access controls, and sufficient audit trails, which are necessary for regulatory compliance, resulting in a high risk of non-compliance and catastrophic security vulnerabilities.
III. Option 2: The Hidden Friction of Buying COTS (The Vendor Lock-in Problem)
The Buy decision is meant to be the safe harbor. You pay the subscription and outsource the maintenance and compliance burden. In reality, you trade one set of unpredictable costs for another: high customization fees and chronic lack of flexibility.
- The High Cost of Out-of-the-Box Customization
COTS solutions, by necessity, are built for the average lab. But your lab is not average; your workflows are your competitive advantage.- Customization Fees: Most specialized genomics LIMS platforms are not truly out-of-the-box. Custom workflow development, specialized instrument connectivity, and data migration require expensive professional services, which often charge premium rates. This expertise can substantially increase the total first-year investment, usually extending implementation timelines past six months.
- Vendor Lock-in: This is the most damaging strategic cost. COTS platforms often use proprietary data models. The cost of migrating away from the vendor later due to service degradation or price increases becomes prohibitively high. You are financially trapped in an inferior product or service, regardless of your lab's evolving needs.
- The Clinical Integration Bottleneck: Why Your LIMS Stops at the EHR Door
The biggest operational constraint of COTS solutions is their failure to solve the last mile of clinical integration.- The Problem: Most commercial EHR systems do not systematically integrate genetic or genomic data. COTS LIMS platforms typically offer only basic, outdated HL7 integration, which is insufficient for clinical decision support.
- The Friction: Your physicians are forced to access reports manually and cross-reference them outside the patient's record, which creates errors, slows diagnosis, and undermines the entire investment. This requires you to build a costly custom EHR integration layer yourself, effectively building the one piece of infrastructure you thought you were buying.

IV. The Only Viable Path: Strategic Partnering (The Predictable TCO Model)
The mature, experienced choice is neither pure Build nor pure Buy, but a Strategic Partner approach. This hybrid model leverages the strengths of COTS for utility functions while dedicating custom, specialized engineering to your core IP and high-friction integration points.
NonStop's core mandate is to help you build only what gives you a strategic edge, and outsource the rest.
- The Core IP Matrix: Build Only What Differentiates You
We use a Core Competency Matrix to define where your capital must be spent ruthlessly. If the process is a generic utility (e.g., user access management), you should partner with a vendor for a pre-validated, certified component.
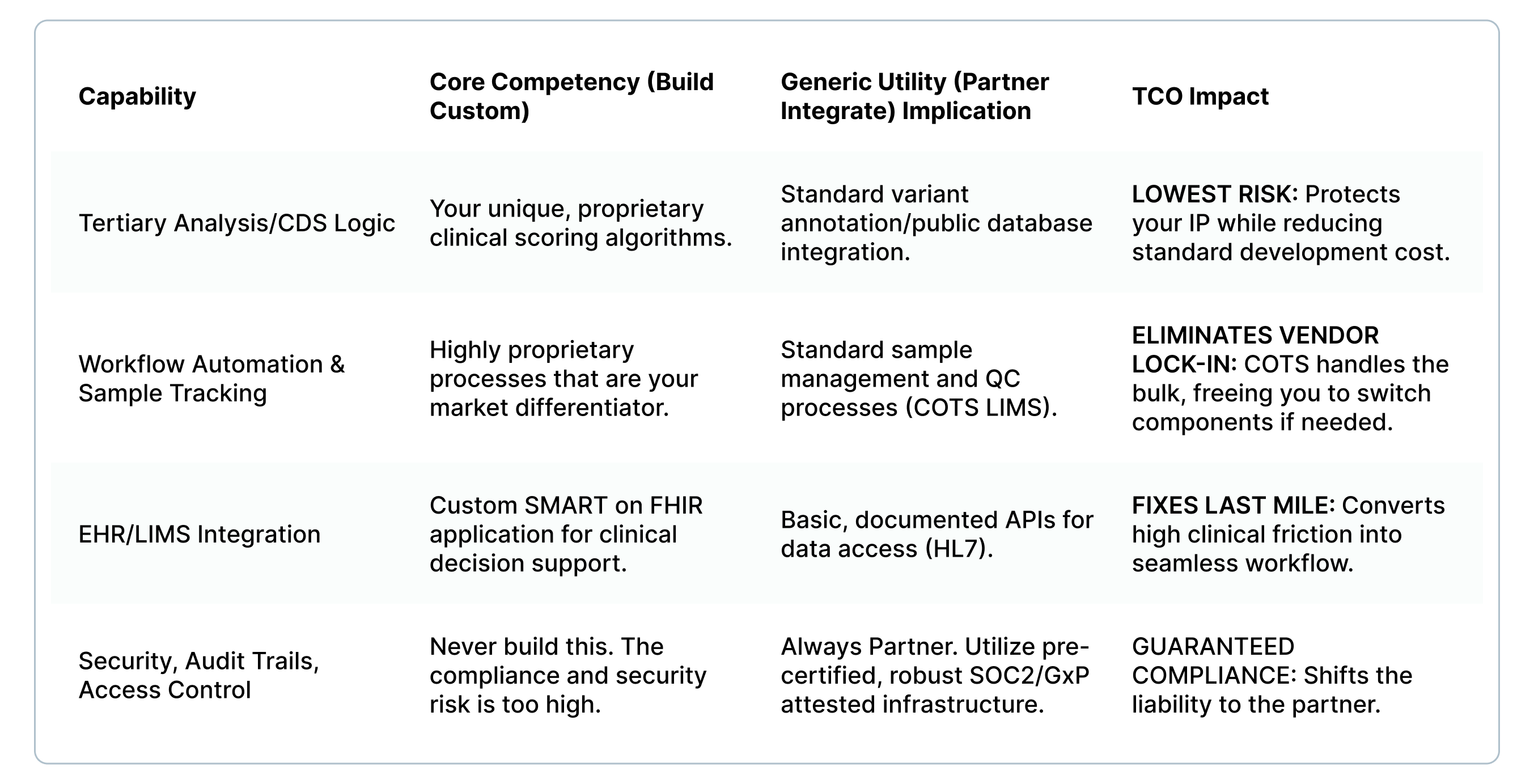
- NonStop's Mandate: Reducing Your Risk and Time-to-Result
We are not just outsourced coders. We are digital product engineers who deeply understand the scientific, regulatory, and financial pressures you face. Our expertise is focused on solving the exact friction points that inflate your TCO:- Predictable TCO: We convert the unpredictable costs of internal staff time, bug fixes, and knowledge loss into fixed-scope, outcome-driven engagements for the custom components you need.
- Clinical Interoperability Expertise: We specialize in eliminating the EHR bottleneck by engineering custom SMART on FHIR integration layers. This ensures your genomic report data is securely and seamlessly accessible to the physician at the point of care.
- Compliance-by-Design: With over a decade of experience building regulated platforms, we integrate GxP, HIPAA, and SOC 2 requirements into the architecture and deployment pipeline from Day 1. Auditability becomes automated, not an expensive, late-stage panic.
V. How We Engineer for Predictable TCO (Our Playbook)
A predictable TCO means building software that is fundamentally resilient to change, scale, and audit scrutiny. This requires a modern, cloud-native engineering philosophy.
- Compliance-by-Design: Automating Your Audits
The single greatest operational cost is manual compliance validation. We reduce this burden through automation:
Automated Compliance Pipeline (DevOps/CI/CD):
We use a DevOps and CI/CD (Continuous Integration/Continuous Delivery) pipeline to automate time-consuming tasks for GxP and SOC 2 audits.
.png)
Automation reduces manual effort, reduces associated workforce costs, and minimizes the risk of human error during crucial audits.
- Eliminating Vendor Lock-in and Silos
We ensure your architecture provides strategic freedom and cost flexibility by adhering to cloud-native best practices:- Cloud-Agnostic Tools: We design platforms using containerization (Docker) and IaC. This minimizes reliance on any single cloud provider (AWS, Azure, GCP) and provides cost-effective alternatives for compute and storage. You maintain strategic freedom.
- Modular Architecture: Components (e.g., LIMS, Analysis, Reporting) are loosely coupled microservices. This means you can update, scale, or replace a specific component, like switching out a sequencing vendor's pipeline, without crashing or revalidating the entire system.

VI. Case in Point: Fixing the Last Mile (Mini-Story)
We recently worked with an innovative liquid biopsy startup that had built a scientifically brilliant secondary analysis platform. They had the science right, but they faced a TCO crisis at the clinical interface.
Their existing, purchased LIMS system produced the clinical report, but getting that data into the hospital EHR was manual, complex, and risked non-compliance. Physicians refused to use the separate external portal. The entire multi-million-dollar investment stalled due to a last-mile integration gap.
The NonStop Solution: We scoped a focused project to build a single, modular SMART on FHIR application. This was a piece of custom IP built exclusively to solve the integration problem. It didn't replace the LIMS; it simply served as a secure bridge that launched directly from the physician's EHR interface, presenting the complex genomic data in a streamlined, workflow-integrated view.
The Outcome: The lab achieved clinical adoption in under 4 months, eliminating manual errors and immediately increasing the value of its core LIMS investment. They strategically built only the custom component that mattered for clinical integration, while leveraging the COTS LIMS for utility. This hybrid focus drastically reduced their operational TCO and accelerated their time-to-market.
VII. Avoiding the Five Most Costly Mistakes
Based on our experience helping organizations through these transformations, here are the five most common pitfalls that inflate TCO and how to avoid them:

VIII. Conclusion
The complexity of genomic data demands a software solution that is predictable, auditable, and scalable. The decision is no longer about choosing between the high development cost of Build or the high strategic cost of Buy. It's about implementing a Strategic Partner model that systematically mitigates risk and ensures compliance from day one.
This approach focused on building minimal custom IP while leveraging specialized partners for compliance automation and complex clinical integration is the fastest, most reliable path to achieving the lowest TCO and the highest return on your digital product investment.
If your team is exploring modernizing LIMS workflows, building cloud-native genomics tools, or integrating EHR/LIMS systems with AI and built-in compliance, NonStop is always open to a conversation. We've spent over a decade helping genomics and healthcare organizations design, engineer, and scale platforms that last.




